Synchrotron radiation

Synchrotron radiation (SR) is the electromagnetic radiation emitted when ultrarelativistic charged particles (usually electrons) are accelerated radially through magnetic fields. Depending on the charachteristics of the source (synchrotron facility), it can consist of soft (< 5 keV) or hard (> 5 keV) x-rays. With respect to x-rays produced by laboratory sources, SR has several peculiar features that can be exploited by different investigation techniques:
- Broad Spectrum (from microwaves to hard X-rays): the users can select the wavelength required for their experiment;
- High Flux: high intensity photon beam allows rapid experiments and high sensitivity (i.e. suitable low elemental concentration);
- High Brilliance: highly collimated photon beam generated by a small divergence and small size source (spatial coherence);
- High Stability: submicron source stability;
- Linear and circular polarization of the x-ray beam;
- Pulsed Time Structure: pulsed length down to tens of picoseconds allows the resolution of process on the same time scale.
Some of the above properties, such as high flux and brilliance, high stability, and spatial coherence, allow pushing some of the SR-based techniques used in this research project to very high performances in terms of spatial resolution, sensitivity, and quality of the data. These techniques are briefly decribed in the following.
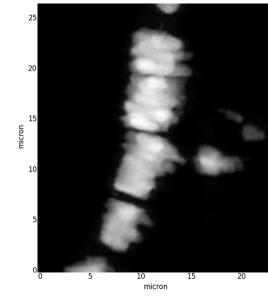
Unlike conventional x-ray imaging, phase-contrast imaging relies on the changes in the real part of the refractive x-rays index as they pass through different tissues, and is much more sensitive than conventional absorption x-ray imaging [1]. The principle of microtomography is very similar to that of medical scanners. It consists in recording a large series of radiographs for different angular positions of the sample. Laboratory microtomographs exist, but the best images, in terms of spatial resolution, signal-to-noise ratio, and quantitative exploitation, are obtained using synchrotron radiation. The high intensity, practically parallel and monochromatic incoming x-ray beam results in a spatial resolution that can reach 30 nm (ID16A at the ESRF). Phase-contrast CµT has been successfully applied to reveal early tumour stages or abnormal neuronal activity [2],[3]. In the present project, it will allow revealing the location and distribution of asbestos bodies in the alveolar network of untouched human lungs tissue samples, and their association with the biological tissue. Experiments performing acquisition of phase-contrast and fluorescence tomographs on the very same asbestos bodies are planned. This will allow combining the information of the two techniques (morphology and elemental distribution), and attempting a reliable elemental quantification.
[1] A Bravin et al. X-ray phase-contrast imaging: from pre-clinical applications towards clinics. Phys. Med. Biol. 58 (2013)
[2] F Pfeiffer et al. High-resolution brain tumor visualization using three-dimensional x-ray phase contrast tomography. Phys. Med. Biol. 52 (2007) 6923
[3] M Fratini et al. Simultaneous 3D imaging of the micro-vascular network and the neuronal system in a mouse spinal cord. Sci. Rep. 5 (2015) 8514.
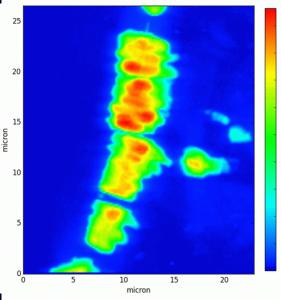
Up to now, the quantification of the elements present in the asbestos bodies has only been performed by bulk analyses, such as inductively coupled plasma mass spectrometry (ICP-MS), which is very sensitive, but completely lack of spatial information, and requires complete digestion of the samples in strong acids that can severely alter their composition. Laboratory tools also usually require large amount of material to detect the most diluted elements, which is rarely available for human samples. On the other hand, synchrotron radiation (SR) micro X-ray fluorescence (µXRF) mapping allows obtaining multi-elemental distribution and concentration with very high lateral resolution (down to few tens of nm) and high sensitivity. FµT is the 3D version of µXRF) mapping. It allows obtaining volumetric (3D) multi-elemental distribution maps. In combination with SR, FµT has high sensitivity (in the low ppm/high ppb range) and a spatial resolution that can reach the nanometer range [1]. In combination with CµT, FµT will allow obtaining reliable, sensitive, and highly spatially resolved 3D multi-elemental distribution and quantification of the asbestos bodies.
[1] S Bohic et al. Biomedical applications of the ESRF synchrotron-based microspectroscopy platform. Journal of Structural Biology 177 (2012) 248-258.
An interesting and long standing question is if the crystallographic structure of asbestos fibres undergoes degradation after prolonged stay in the biological medium. This point is of interest because the degradation of the mineral structure can favour the migration of mineral ions in the cellular tissue, which can, in turn, take part in the biomineralization process, and thus play a role in the carcinogenesis. Laboratory x-ray diffraction is not suitable to study single asbestos fibers due to both the large area of the x-ray beam and to the low intensity that can be reached by laboratory sources. Conversely, synchrotron µXRD allows for small and intense x-ray beam spot sizes.
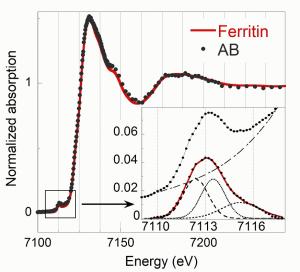
X-ray absorption spectroscopy (XAS) is a chemical selective technique informative on the oxidation state and binding environment of the element of interest. In fact, the absoprtion spectrum obtained by scanning the incident photon energy in a range that includes the absorption energy edge of an inner shell electron of an element of interest, has unique features depending on the oxidation state and binding environment of the selected element. In particular, by comparing the near edge spectrum (rouglhy -50 - +150 eV with respect to the absorption edge energy), also indicated as XANES spectrum (X-ray absorption near edge spectroscopy), with the absorption spectra of reference compounds, it is possible to retrieve the chemical form of the selected element. If the studied system consist of a mixture of compounds of the selected element, it is possible to perform a linear combination fitting of the reference spectra to match the sample spectrum. If the set of reference compounds is representative of the studied system, this procedure allows revealing the speciation of the selected element. In this project, the XANES spectrum of asbestos bodies will be compared with relevant Fe references, such as ferritin, hemin, hematin, hematite..., or with a linear combination of the latter. The figure shows a comparison between the XANES spectrum of an absestos body with one of ferritin, showing a good match.